Theoretical yield formula organic chemistry 342412-How to calculate the theoretical yield in chemistry
This is the theoretical yield, expressed in moles This can also be expressed in units of mass using the literature MW of the product use the mass of product obtained to determine the percent yield percent yield = grams of product obtained X 100% theoretical yield (in grams)From a reaction can be compared to the theoretical yield to give the percentage yield Percentage yield = Actual amount obtained ÷ Theoretical amount possible x 100% In this reaction, suppose the actual yield is 0905 g of ethyl bromideThe theoretical yield refers to the amount that should be form when the limiting reagent is completely consumed The actual yield is expressed as a percentage of the theoretical yield This is called the percent yield To find the actual yield, simply multiply the percentage and theoretical yield together

Reaction Percent Yield Introduction And Practice Exercises
How to calculate the theoretical yield in chemistry
How to calculate the theoretical yield in chemistry-To determine the percent yield Divide the actual yield made in the lab by the calculated theoretical amount, and multiply by 100 For a synthesis – to find the overall percent yield, multiply the individual percent yields of every step by each other (ex 3 steps, all 30% yield – 030 x 030 x 030 = 027 x 100 = 27% overall) Alternatively, you can take the limiting reagent molar amount ofTheoretical yield is the amount of product a reaction would produce if the reactants reacted completely Problem Given the reaction Na 2 S(aq) 2 AgNO 3 (aq) → Ag 2 S(s) 2 NaNO 3 (aq) How many grams of Ag 2 S will form when 394 g of AgNO 3 and an excess of Na 2 S are reacted together?



How To Calculate Percent Yield In Chemistry 15 Steps
Chemistry Stack Exchange Calculating theoretical yield for a reaction with a single product is pretty trivial Multiply the amount of moles of limiting reagent to the molar ratio of the limiting reagent and product to the molecular weight of the product However, if the reaction is also capable of producing side product, this method no longer works because some portion of that limiting reagent would beThe answer is theoretical yield = 6 mol It takes two molecules of NaI to make one molecule of ICH 2 CH 2 CH 2 I 6 Calculate the percentage yield The percent yield is simply the actual yield divided by theoretical yield multiplied by 100Organic chemistry How to calculate theoretical yield if there are minor products?
From the relative formula masses of the reactants The ratio of these amounts is expressed as a percentage yield % Yield = actual yield x 100 theoretical yield Example 230 g of ethanol, CH 3CH 2OH, is used to form the ester ethyl ethanoate, CH 3COOCH 2CH 3, according to the equation CH 3COOH(l) CH 3CH 2OH(l) → CH 3COOCH 2CH 3(l) H 2O(l)From the relative formula masses of the reactants The ratio of these amounts is expressed as a percentage yield % Yield = actual yield x 100 theoretical yield Example 230 g of ethanol, CH 3CH 2OH, is used to form the ester ethyl ethanoate, CH 3COOCH 2CH 3, according to the equation CH 3COOH(l) CH 3CH 2OH(l) → CH 3COOCH 2CH 3(l) H 2O(l)Calculate the maximum theoretical yield of calcium oxide that can be produced from 250 g of calcium carbonate Write down the balanced chemical equation CaCO 3 \(\rightarrow\) CaO CO 2
Feb 11, 17 The calculation using the theoretical yield formula is simple, provided it is done in a methodical manner Feb 11, 17 The calculation using the theoretical yield formula is simple, provided it is done in a methodical manner Saved from buzzlecom A Simple Guide on How to Calculate Theoretical YieldTheoretical Yield Formula The quantity of a product obtained from a reaction is expressed in terms of the yield of the reaction The amount of product predicted by stoichiometry is called the theoretical yield, whereas the amount obtained actually is called the actual yield A reaction yield is reported as the percentage of the theoretical amountTry these practice problems below 1 For the balanced equation shown below, if 938 grams of PCl5 were reacted with 3 grams of H2O, how many grams of H3PO4 would be produced?
/scientist-pouring-iron-chloride-into-beaker-of-potassium-thiocyanate-702545775-59fb5991845b340038498040.jpg)


What Is The Theoretical Yield Of A Reaction



Organic Chemistry Laboratory Green Chemistry Calcu Chegg Com
This video shows you how to calculate the theoretical and percent yield in chemistry The theoretical yield is the maximum amount of product that can be proUse the molar mass of the product to convert moles product to grams of product In equation form grams product = grams reactant x (1 mol reactant/molar mass of reactant) x (mole ratio product/reactant) x (molar mass of product/1 mol product) The theoretical yield of our reaction is calculated using molar mass of H 2 gas = 2 gramsPercent yield = 102% Chemistry Is Everywhere Actual Yields in Drug Synthesis and Purification Many drugs are the product of several steps of chemical synthesis Each step typically occurs with less than 100% yield, so the overall percent yield might be very small



Calculating Theoretical Yield Youtube



Organic Chemistry Lab I Chem 237 Experiment 2 Recrystallization Flashcards Quizlet
To express the efficiency of a reaction, you can calculate the percent yield using this formula %yield = (actual yield/theoretical yield) x 100 A percent yield of 90% means the reaction was 90% efficient, and 10% of the materials were wasted (they failed to react, or their products were not captured) Part 1 Finding the Limiting ReactantA DielsAlder reaction happens between 9Anthracenemethanol (6 g/mol) and NMethylmaleimide ( g/mol) I'm using 0069 g 9Anthracenemethanol (0033 mol) and 011g NMethylemaleimide ( mol, limiting reagent) Since DA reactions have 100% atom economy, the product has the molar mass of both products combined, g/molTo express the efficiency of a reaction, you can calculate the percent yield using this formula %yield = (actual yield/theoretical yield) x 100 A percent yield of 90% means the reaction was 90% efficient, and 10% of the materials were wasted (they failed to react, or their products were not captured) Steps



Calcium Oxide Reacts With Water In A Combi Clutch Prep


Www Studocu Com En Us Document University Of Illinois At Chicago Organic Chemistry Laboratory I Assignments Williamson Ether Synthesis Preparation Of Phenacetin From Acetaminophen View
Theoretical yield (g) =(amount of LR)•(MW of P)• moles of P moles of LR (LR = limiting reagent, P = product, MW = molecular weight in g/mol) Percent yield = purified percent yield = amount of P (g) theoretical yield (g) •100 Percent yield (if stoichiometry is 11) = amount of P (mol) amount of LR (mol) •1003999 g m o l = 1452 g N a B r We see there is expected to be 1452 grams of sodium bromide product To calculate percent yield, you simply take actual yield 1099 grams of sodium bromide, divided by the theoretical yield 1452 grams of sodium bromide 1099 g N a B r 1452 g N a B r ∗ 100 = 7569So, to stop you from wondering how to find theoretical yield, here is the theoretical yield formula mass of product = molecular weight of product * (moles of limiting reagent in reaction * stoichiometry of product)
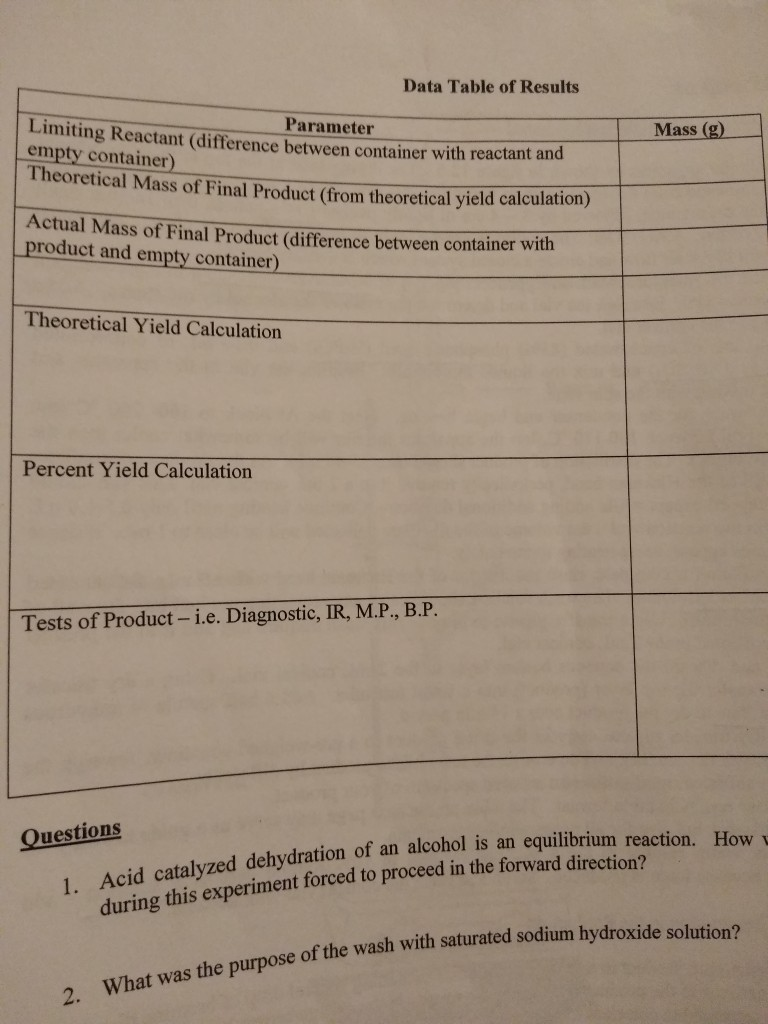


Solved In The Organic Chemistry Lab Dichlorocarbene Addi Chegg Com


Http Cdochemistrychristman Pbworks Com W File Fetch Ap chemistry unit 1 13 14 87 answers Pdf
You expect to create six times as many moles of carbon dioxide as you have of glucose to begin with The theoretical yield of carbon dioxide is (0139 moles glucose) x (6 moles carbon dioxide / mole glucose) = 04 moles carbon dioxideTheoretical yield calculations and multiply by 100 to calculate the percent yield Example Assume 296g of salicylic acid was obtained experimentally 296 X 100 = 795%Theoretical yield of NaCl in grams = theoretical yield in moles × molar mass of NaCl Theoretical yield of NaCl in grams = 017 moles of NaCl × 5844 g/mole Theoretical yield of NaCl in grams = 993 grams Step 5 Find the Percentage Yield If you actually carry out this reaction in a lab, you will be able to find the actual yield of the reaction


Thiols And Thioethers Master Organic Chemistry
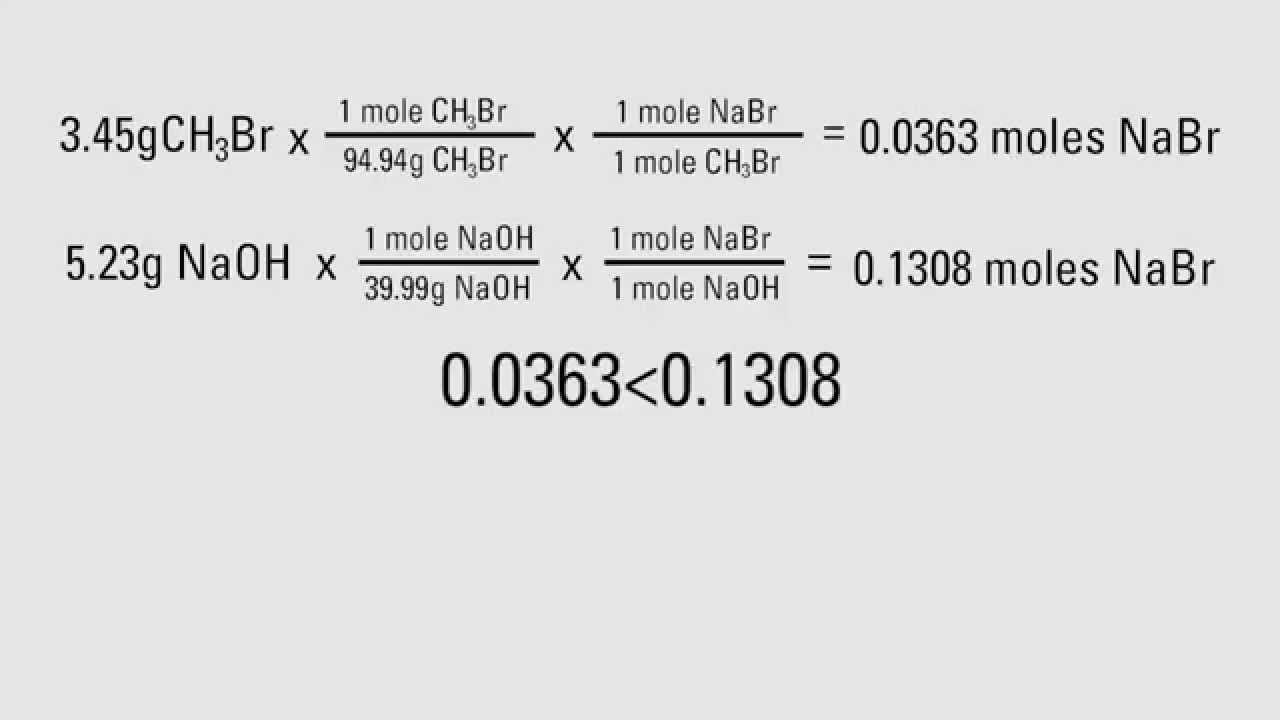


How To Calculate Theoretical Yields Youtube
The percent yield is the ratio of the actual yield to the theoretical yield, expressed as a percentage (1291) Percent Yield = Actual Yield Theoretical Yield × 100 % Percent yield is very important in the manufacture of products Much time and money is spent improving the percent yield for chemical productionYield Data Theoretical yield of the two organic products = moles of limiting reagent (cisand trans 2methylcyclohexanol) * MolW of 1methlcyclohexene or(3methylcycolhexene) = *9617 = 3914g Theoretical yield of water = *18 = 0733gSo, to stop you from wondering how to find theoretical yield, here is the theoretical yield formula mass of product = molecular weight of product * (moles of limiting reagent in reaction * stoichiometry of product)



How To Calculate Theoretical Yield 12 Steps With Pictures



1 Percent Yield Chapter Theoretical Actual And Percent Yield Theoretical Yield The Maximum Amount Of Product Calculated Using The Balanced Equation Ppt Download
Formula for percentage yield Should it be percentage yield = actual yield theoretical yield × 100 {\displaystyle {\mbox{percentage yield}}={\frac {\mbox{actual yield}}{\mbox{theoretical yield}}}\times 100}Theoretical yield = 1 g;Eg, volume, if the product is a gas)



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com


Www Unf Edu Michael Lufaso Chem45 Chapter3 Pdf
Organic chemistry How to calculate theoretical yield if there are minor products?A DielsAlder reaction happens between 9Anthracenemethanol (6 g/mol) and NMethylmaleimide ( g/mol) I'm using 0069 g 9Anthracenemethanol (0033 mol) and 011g NMethylemaleimide ( mol, limiting reagent) Since DA reactions have 100% atom economy, the product has the molar mass of both products combined, g/molLearn how to identify the limiting reactant in a chemical reaction and use this information to calculate the theoretical and percent yields for the reaction If you're seeing this message, it means we're having trouble loading external resources on our website
/GettyImages-82845115-1--56a134e33df78cf772686225.jpg)


Stoichiometry Definition In Chemistry
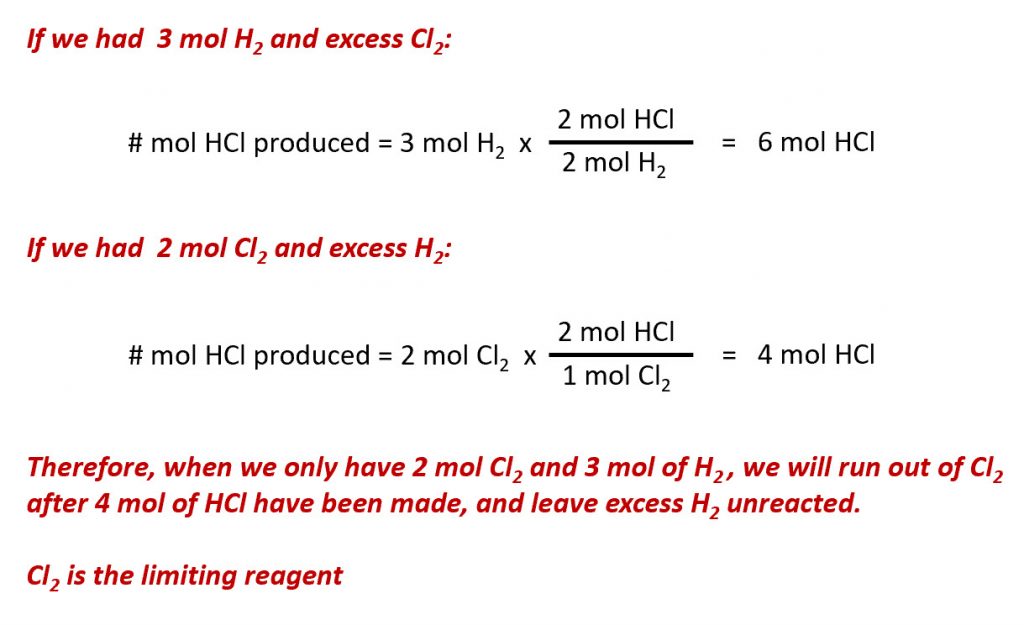


Ch150 Chapter 6 Quantities In Chemistry Chemistry
Your percent yield would be (33/36) x 100 = 92% If, in the propane combustion example, you collected only 47 moles of the 6 moles produced by the reaction, your theoretical yield would be (47/6Divide number of AI moles by two, since it takes four to make 2 AI2O3) Finally, to get a theoretical yield, you'll need to multiply the number of moles of the product by the molecular weight of the product (Ex 05 moles of AI2O3 multiplied by the molecular weight of AI2O3 is approximately grams per moleGeneral chemistry students can use this site to learn about basic green chemistry calculations such as atom economy, limiting reactant, theoretical yield, and percent yield Organic chemistry students can use this site to prepare "prelab" reports that summarize the number of moles and mass of reactants and products



Theoretical Actual And Percent Yield Problems Chemistry Tutorial Youtube



1 Write The Reaction Equation 2 Write A Sample T Chegg Com
Theoretical Yield Formula In a chemical reaction the maximum amount of product formed is determined by the amount of limiting reactant that is used up Stoichiometry is used to predict this amount of product It is known as the theoretical yield12 g is the theoretical yield 5 g is the actual yield 6 Calculate the PERCENT YIELD The percent yield is based upon the theoretical yield actual yield (g) 5 g x 100 % = Percent Yield = x 100 % = 68% theoretical yield (g) 1216 g (For theoretical yield, carry more sig figs in this calculation)Chemists have to be concerned with just how completely their reactants react to form products To compare the amount of product obtained from a reaction with the amount that should have been obtained, they use percent yield You determine percent yield of a chemical reaction with the following formula Lovely, but what is an actual



Calculating Percent Recovery Percent Yield



Calculate The Theoretical Yield To Determine The Yield In A Chemical Reaction Youtube
How to calculate the theoretical yield of a DielsAlder reaction?PCl54H2O=>H3PO45HCl 2 For the balanced equation shown below, if 1 grams of C2H5Cl were reacted with 373 grams of O2, how many grams of Cl2 would be produced?Since your actual yield is in grams, we'll convert the theoretical yield into grams as well Molar mass SrCO3 = Molar mass of Sr Molar mass of C 3 (Molar mass of O) 8762 11 3(1600) =


Calculation Of Theoretical Yield Organic Chemistry I 212 01
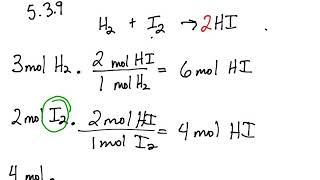


5 3 Calculating Reaction Yields Problems Chemistry Libretexts
Based on that value, you can find the percentage yield by using the ratio of the actual yield and the theoretical yield The formula for calculating the percent yield is Percentage yield = mass of actual yield ÷ mass of theoretical yield × 100% Let's assume that you obtained an actual yield of 850 gramsChemistry Stack Exchange 0 Calculating theoretical yield for a reaction with a single product is pretty trivial Multiply the amount of moles of limiting reagent to the molar ratio of the limiting reagent and product to the molecular weight of the productChemists have to be concerned with just how completely their reactants react to form products To compare the amount of product obtained from a reaction with the amount that should have been obtained, they use percent yield You determine percent yield of a chemical reaction with the following formula Lovely, but what is an actual
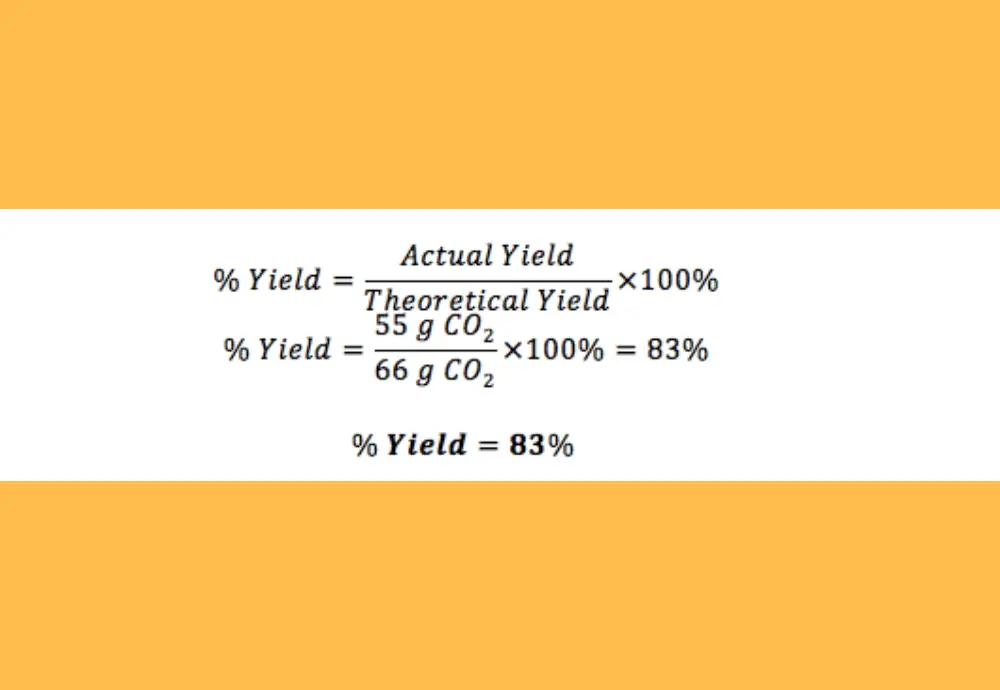


Percent Yield Calculator 100 Free Calculators Io


Solved 33 Chemistr Organic Chemistry Lab Manual 4 Ch Chegg Com
Theoretical Yield in the Combustion of Propane The combustion of propane (C3H8) forms carbon dioxide (CO2) and water (H2O) according to the chemical equation below Think of this equation asYield}$$\times$ 100% Percentage yield = $\frac{06}{14}$$\times$ 100% Percentage yield = 429% The percentage yield of this reaction is 429%, Scientist tries to choose reactions with a high percentage yield or high atom economySubstitute the values in the corresponding formula Percentage yield = $\frac{Actual\;



Reaction Percent Yield Introduction And Practice Exercises



Reaction Yield An Overview Sciencedirect Topics
The theoretical yield refers to the amount that should be form when the limiting reagent is completely consumed The actual yield is expressed as a percentage of the theoretical yield This is called the percent yield To find the actual yield, simply multiply the percentage and theoretical yield togetherWant to master theoretical yield?This is also called the "theoretical yield" The percent yield is found by dividing the actual mass of product isolated, the "actual yield," which is given in the problem to be 0484 grams, by the



Quantitative Chemistry Secondary Science 4 All


Preparation Recrystallization Of Acetanilide Mendelset
The theoretical yield of 100 g is the calculated amount of product, assuming that the reaction is 100% efficient The percentage yield is the ratio of actual yield to theoretical yield expressed as a percentage (37 g/100 g) × 100% = 37% 3 Formula for percentage yieldTo calculate the theoretical yield, consider the reaction (2) CO ( g) 2 H 2 ( g) → CH 3 OH ( l) (3) 280 40 3 ( stoichiometric masses in g, kg, or tons) 12 t o n s H 2 × 3 C H 3 O H 40 H 2 = 96 t o n s C H 3 O H Thus, the theoretical yield from 12 metric tons (12x10 6 g) of hydrogen gas is 96 tonsFrom a reaction can be compared to the theoretical yield to give the percentage yield Percentage yield = Actual amount obtained ÷ Theoretical amount possible x 100% In this reaction, suppose the actual yield is 0905 g of ethyl bromide



How To Calculate The Theoretical Yield And Percentage Yield In A Balanced Chemical Equation Quora



Reaction Percent Yield Introduction And Practice Exercises
How to calculate the theoretical yield of a DielsAlder reaction?By comparing the amounts of product we state that the theoretical yield, sometimes referred to as the 100% yield, represents the smaller amount Therefore the theoretical yield is 426 g NH3The extent to which a reaction's theoretical yield is achieved is commonly expressed as its percent yield percent yield = actual yield theoretical yield × 100 % Actual and theoretical yields may be expressed as masses or molar amounts (or any other appropriate property;


Chemistry 104 Synthesis Of Aspirin
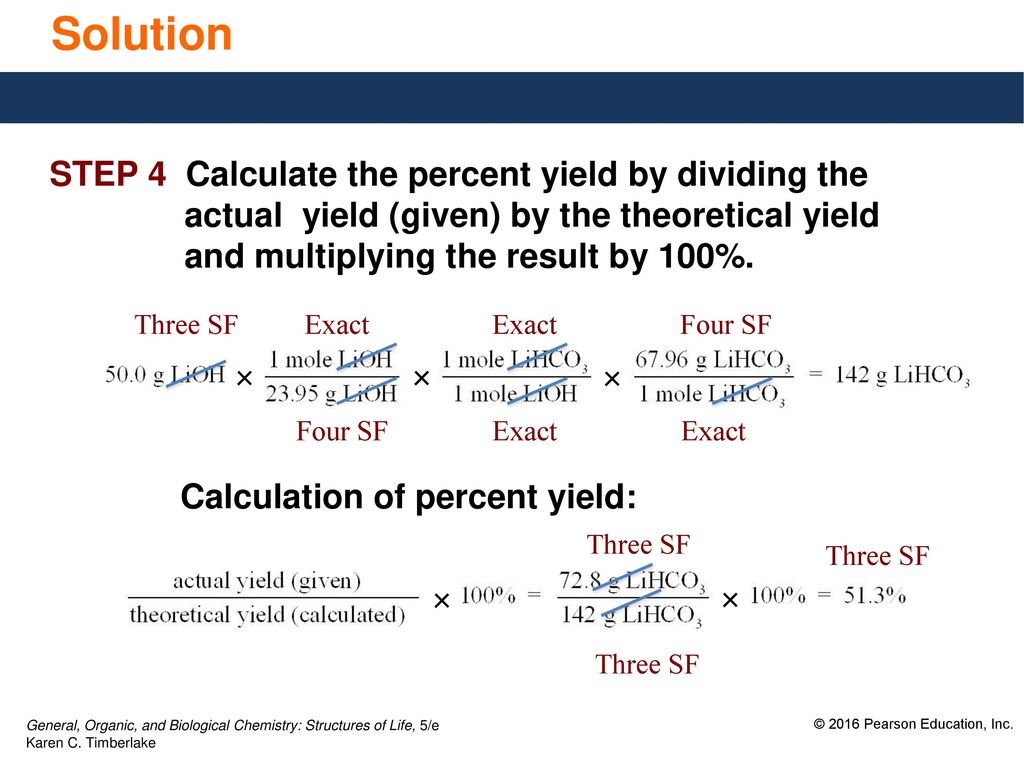


7 8 Limiting Reactants Percent Yield Ppt Download
Theoretical Yield 2Bromo1Hydroxy1Methylcyclohexane C 7 H 13 BrO 198 N/A N/AJan 09, 21 Theoretical yield refers to how much product will be produced with This is a strategy to use when calculating the theoretical yield of a chemical reaction P W G (1996) Vogel's Textbook of Practical Organic Chemistry (5th What Is the Theoretical Yield of a Reaction?



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com


Q Tbn And9gctje7pkewyqa0nnrn06zpgzepy Yorrmegghf1btx8a49kkxzp8 Usqp Cau



Reaction Yield Protocol


Williamson Ether Synthesis Mendelset
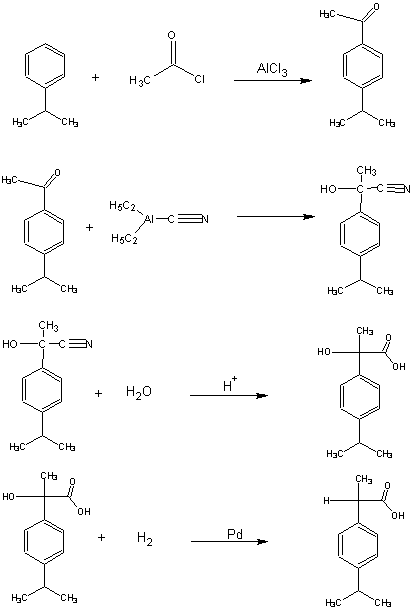


Green Chemistry English Green Chemistry
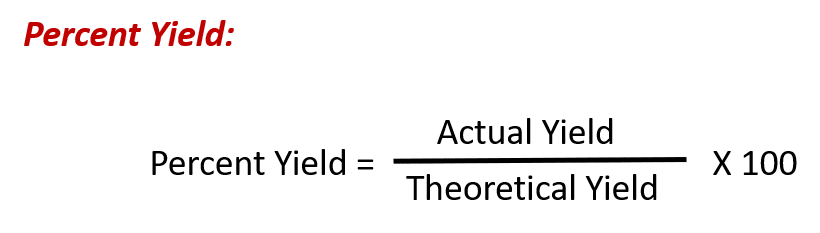


Ch150 Chapter 6 Quantities In Chemistry Chemistry



Oneclass 1 For This Experiment What Is The Theoretical Yield Balanced Chemical Equation Mechanism
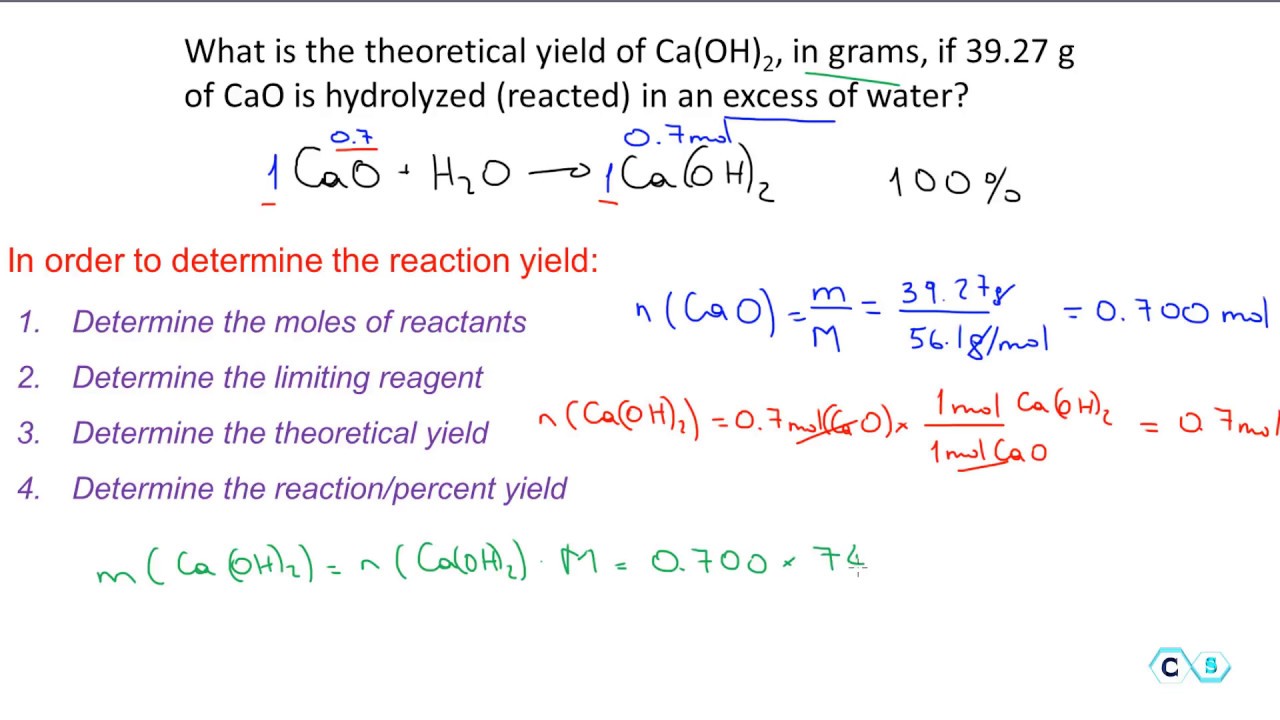


Stoichiometry Percent Yield Practice Problem 1 Youtube



How To Calculate Percent Yield In Chemistry 15 Steps


3



General Organic And Biological Chemistry Fourth Edition Karen Timberlake 6 8 Percent Yield And Limiting Reactants Chapter 6 Chemical Reactions And Quantities Ppt Download



Percent Yield Chemistry Video Clutch Prep



How To Calculate Percent Yield In Chemistry Chemistry Science Chemistry Physical Chemistry



Howto How To Find Percent Yield Without Actual Yield



M Sc Inorganic Chemistry Laboratory Manual Complex Preparations



Synthesis Of Barbituric Acid From Urea And Dimethyl Malonate Labmonk
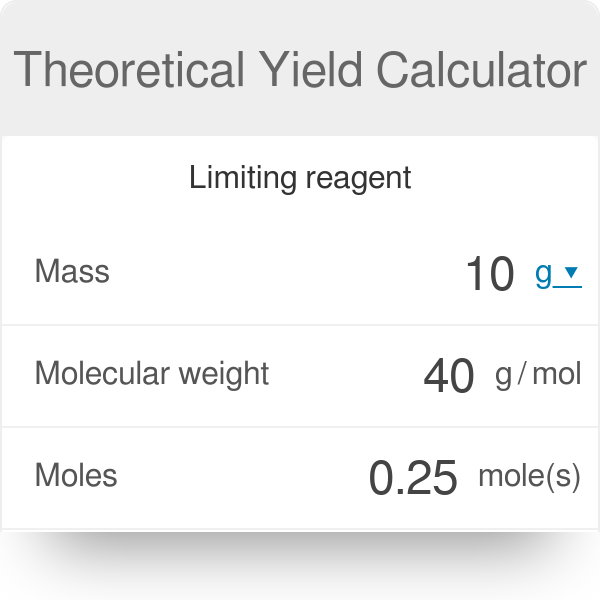


Theoretical Yield Calculator



Theoretical Actual Percent Yield Error Limiting Reagent And Excess Reactant That Remains Youtube



Theoretical Yield Calculator



Theoretical Yield Youtube


Calculating Percentage Yield



Reaction Yield Protocol



Solved How To Calculuate The Theoretical And Actual Yield Chegg Com



Organic Chemistry Calculate The Theoretical Yield In The Production Of T Pentyl Chloride Synthesis If T Pentyl Alcohol Homeworklib


Www Unm Edu Orgchem 303l pages 05 lab 2 ethanol Pdf



Green Chemistry For Chemical Synthesis Pnas


Www Tsijournals Com Articles The Grignard Synthesis Of Triphenylmethanol Pdf
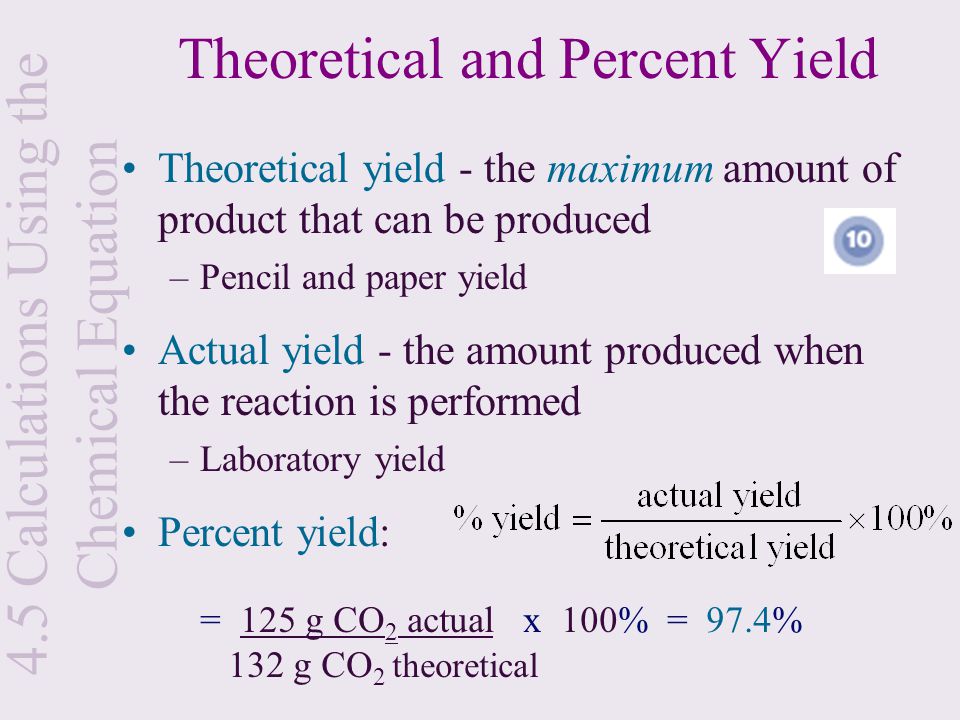


How To Calculate Percentage Yield In Chemistry How To Wiki
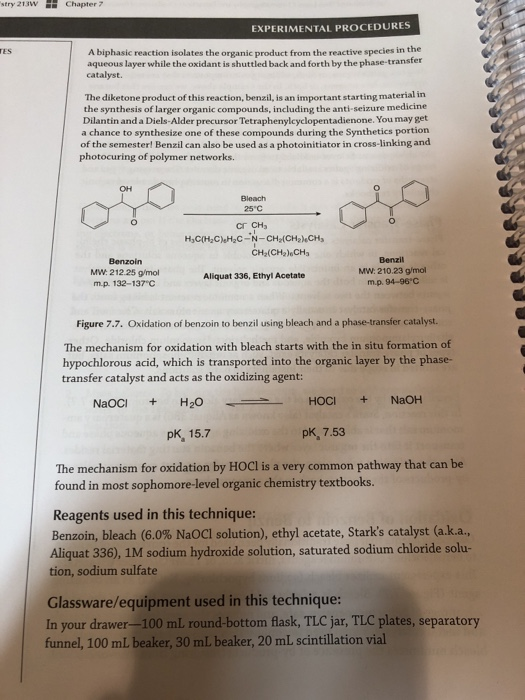


Solved Using The Balanced Chemical Equation On Pg 174 Pi Chegg Com


Q Tbn And9gcrogmlnxe Ntkkzpcy5dmfkqojpkz0ksqodth4epgbawy Mtqxy Usqp Cau



Calculating Actual Yield Given The Percent Yield Youtube


Green Chemistry


Chemistry Atom Economy And Percentage Yield



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com


Chemistry Atom Economy And Percentage Yield
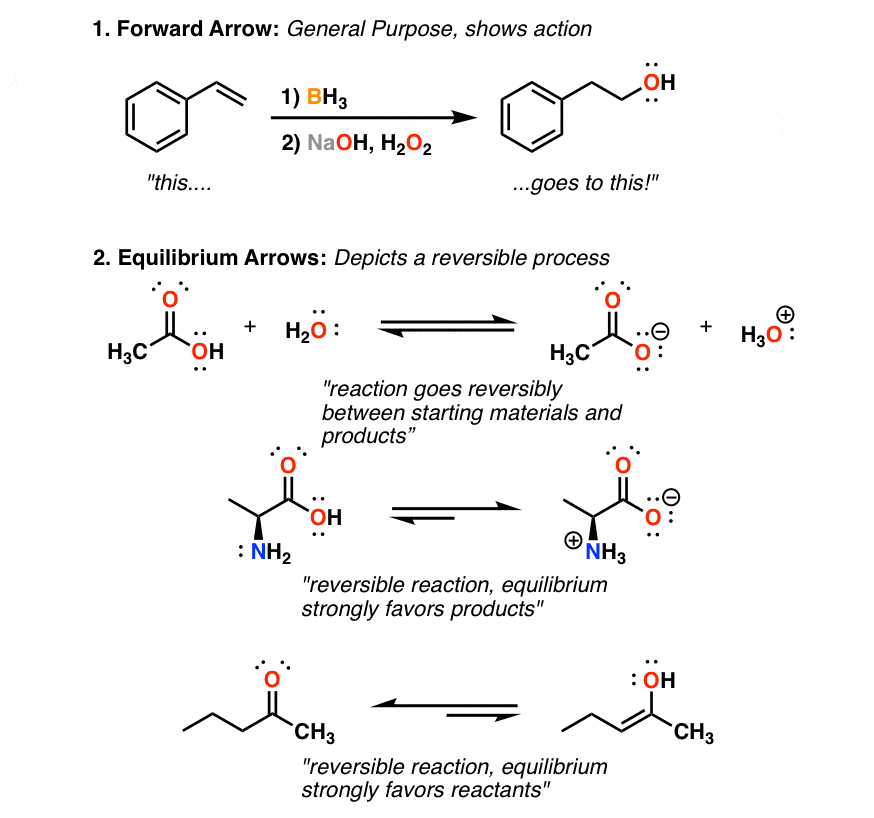


The 8 Types Of Arrows In Organic Chemistry Explained Master Organic Chemistry



Image Result For Theoretical Yield School Work Chemistry Basic Concepts



Overcoming Organic Chemistry Synthesis Of Salicylic Acid
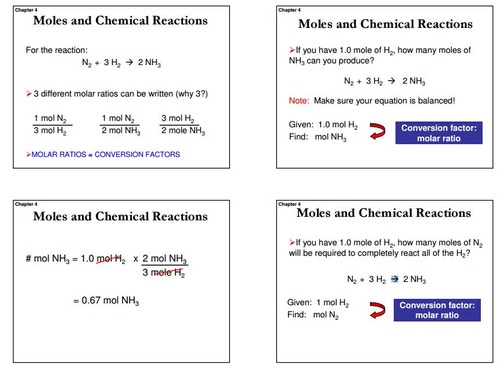


Chemistry Chapter 6 Flashcards Quizlet
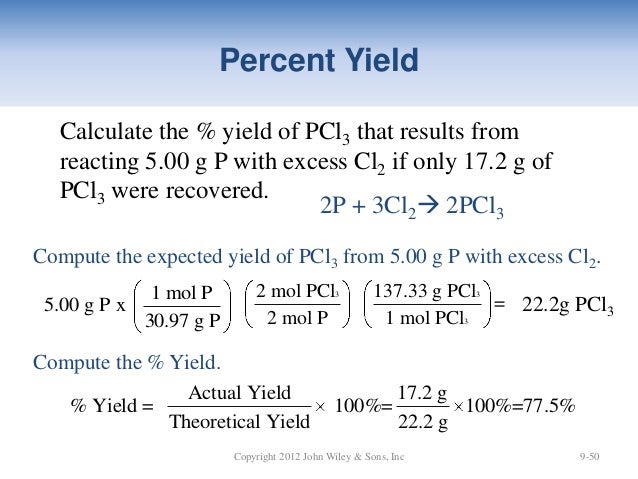


Nwtc General Chemistry Ch 09


Calculation Of Theoretical Yield Organic Chemistry I 212 01



Howto How To Find Percentage Yield In Chemistry



Yield Calculations Faculty Staff Sites



Percent Yield Tutorial Explained Practice Problems Crash Chemistry Academy Youtube



Xperiment 7 Organic Chemistry L Aldol Condensati Chegg Com


Organic Chemistry I Lab Labarchives Your Electronic Lab Notebook
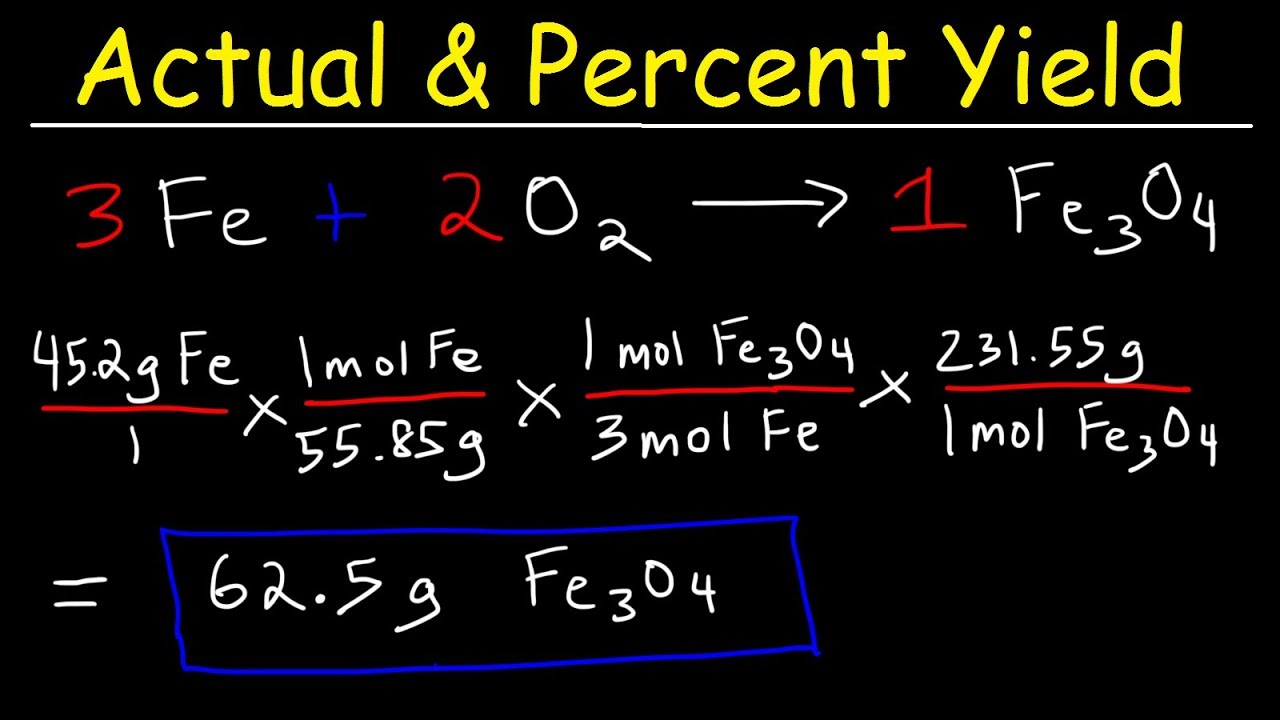


How To Calculate The Percent Yield And Theoretical Yield Youtube


Q Tbn And9gcs60s5akezigys4bjyqzve5tdrkwvatrchodzxvxw7 9iinr194 Usqp Cau



Percent Yield Percent Purity Solutions Examples Videos


2
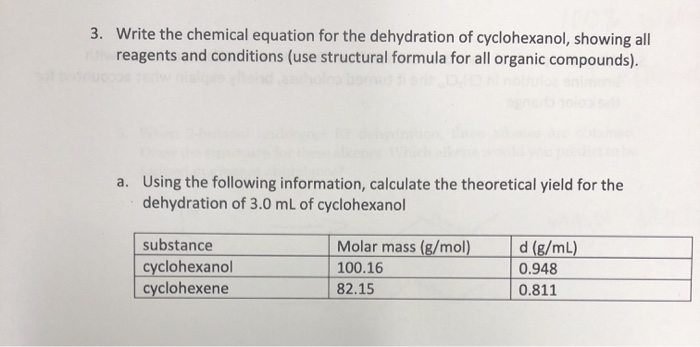


Solved 3 Write The Chemical Equation For The Dehydration Chegg Com



Stoichiometry Chemistry Video Clutch Prep



College Organic Chem Lab Ethanol Fermentation Not Sure Where I M Going Wrong Here My Theoretical Is Smaller Than My Actual Values From Question 2 Came From Table In Book They Gave Us
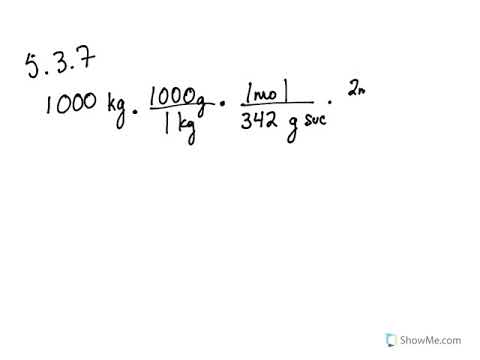


5 3 Calculating Reaction Yields Problems Chemistry Libretexts


Www Imedpub Com Articles Atom Economy In Drug Synthesis Is A Playground Of Functional Groups Pdf



Oneclass Calculate The Theoretical Yield Of C6h5no2 For This Reaction Once You Convert 28 0 G Hnos A



How To Calculate Theoretical Yield And Percent Yield Youtube



Synthesis Of Benzil From Benzoin Labmonk



A Simple Guide On How To Calculate Theoretical Yield Detox Fast Wine Folly Wine Map



Reaction Yield Protocol



Solution Urea Ch4n2o Is A Common Fertil Clutch Prep



Calculating Radical Yields Organic Chemistry Video Clutch Prep



Magnesium Oxide Percent Yield Lab Report Schoolworkhelper



College Organic Chem Lab Ethanol Fermentation Not Sure Where I M Going Wrong Here My Theoretical Is Smaller Than My Actual Values From Question 2 Came From Table In Book They Gave Us



Calculating Percent Yield
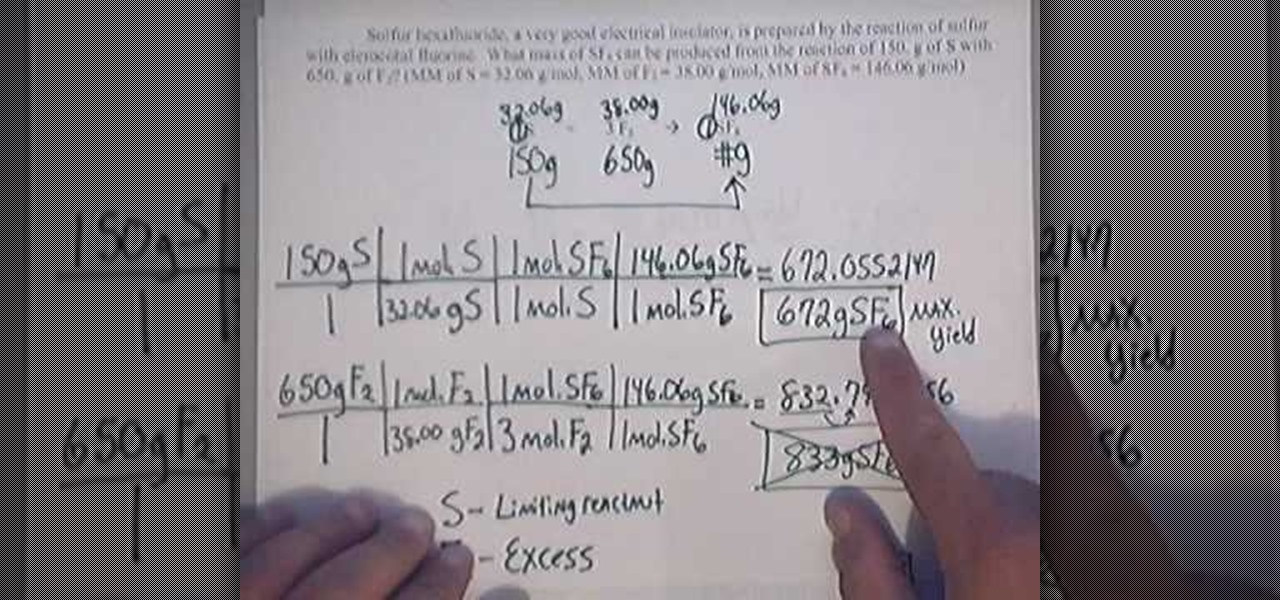


How To Calculate Percent Yield Math Wonderhowto



Reaction Percent Yield Introduction And Practice Exercises
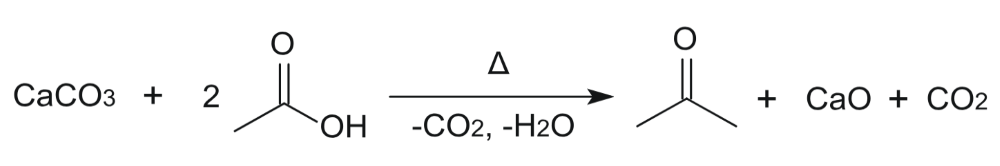


Theoretical Yield Calculator


8 6 Limiting Reactant Theoretical Yield And Percent Yield From Initial Masses Of Reactants Chemistry Libretexts



How To Find Actual Yield Theoretical Yield And Percent Yield Examples Practice Problems Youtube
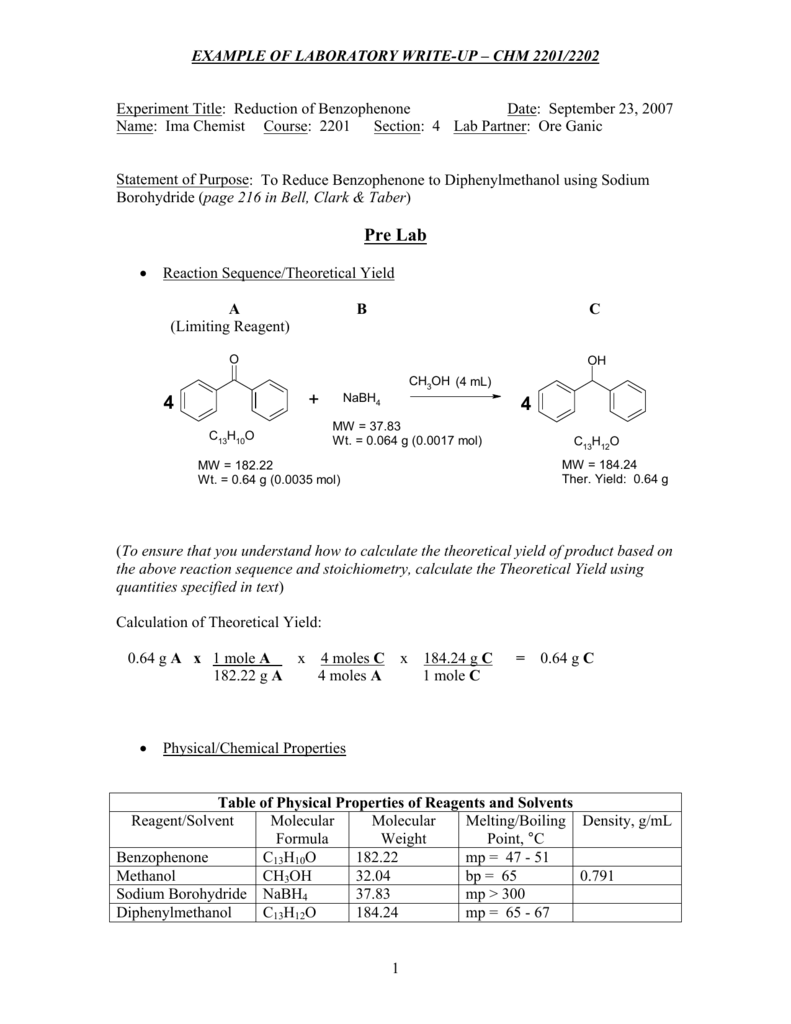


Experiment Title Reduction Of Benzophenone Date September 23



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com


コメント
コメントを投稿